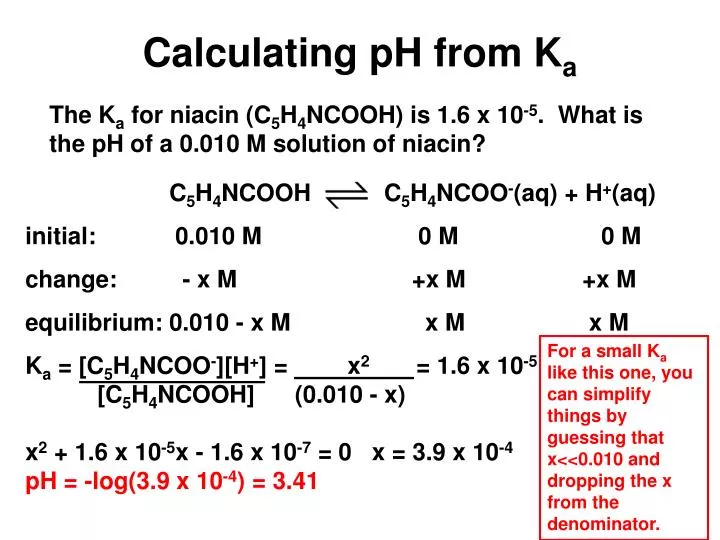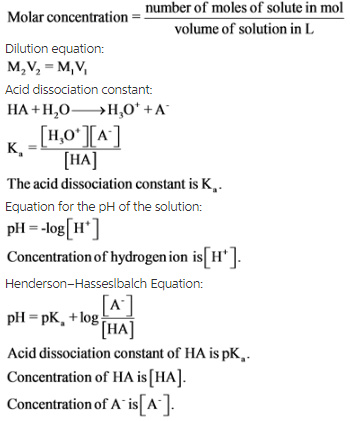
The Ka value for acetic acid, CH3COOH(aq), is 1.8x10^-5. Calculate the ph of a 2.80 M acetic acid solution - Home Work Help - Learn CBSE Forum
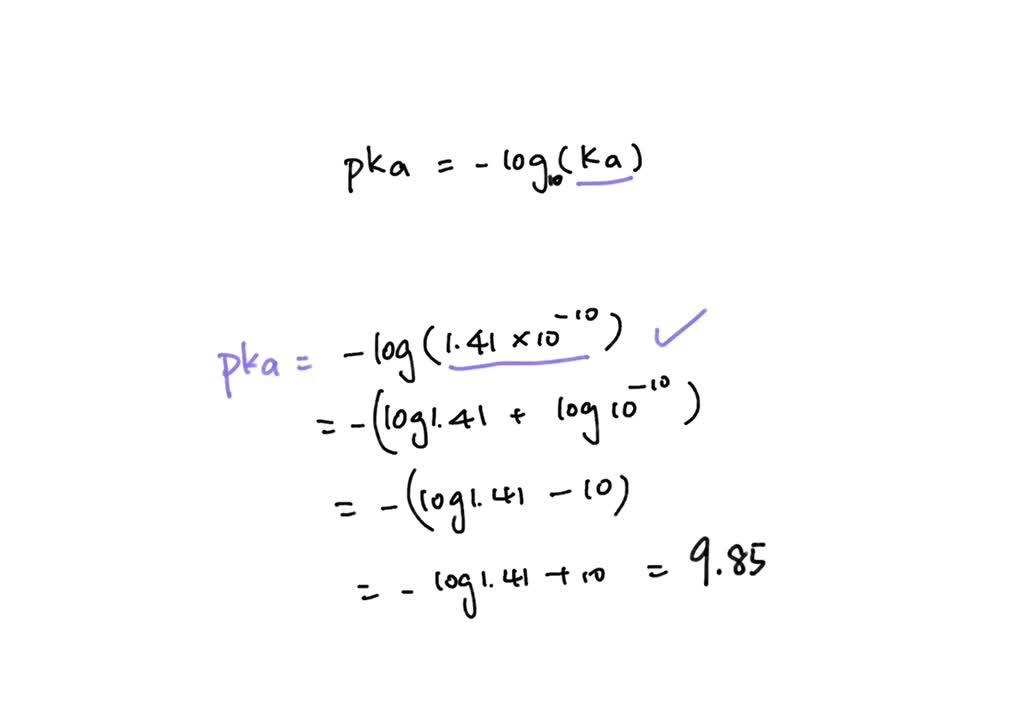
SOLVED: How would you calculate the pka or ka for CH2ClCH2COOH and CH2FCH2COOH if you only knew that this is a carboxylic acid? Do you write the conjugate acid and base to


![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)

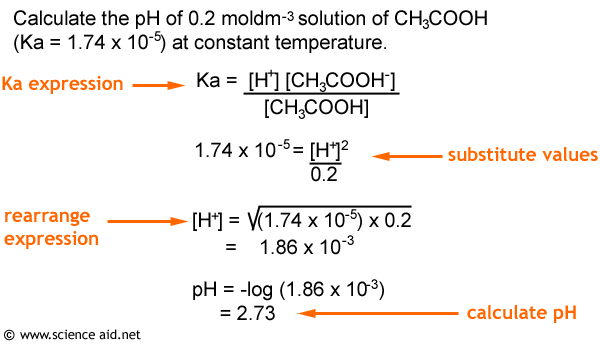
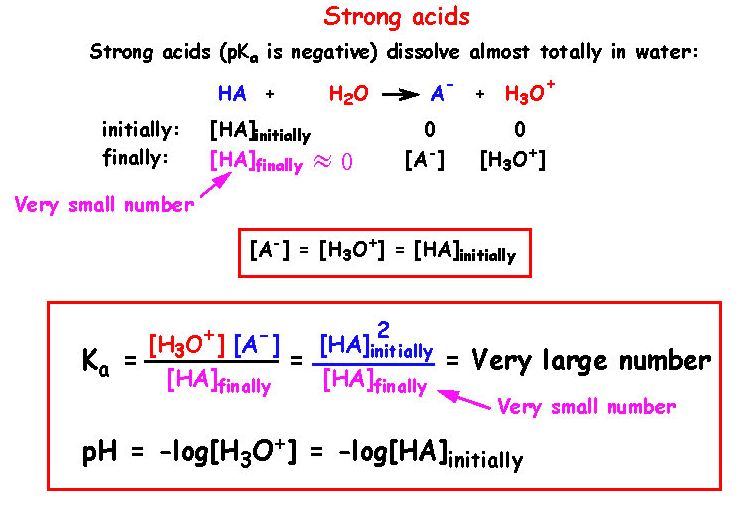
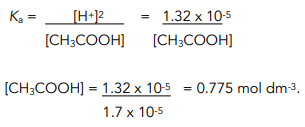
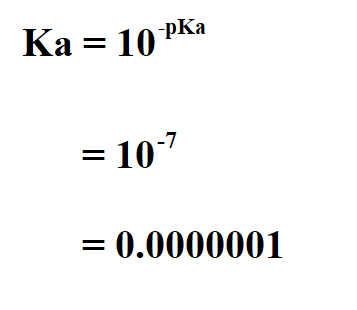


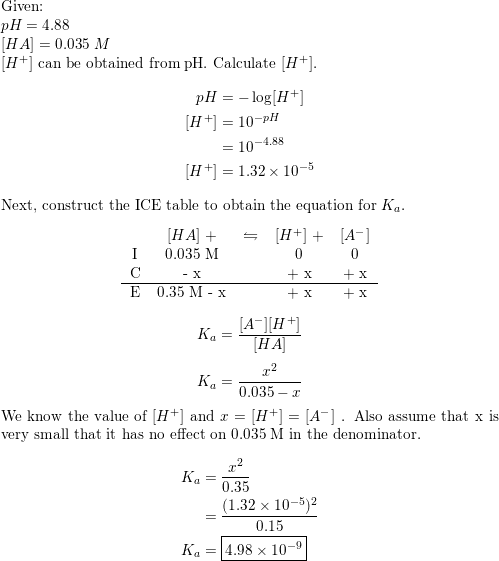


![Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com Solved 3. Calculate the [H3O+], [OH-], pH and pOH of a 0.120 | Chegg.com](https://media.cheggcdn.com/study/f5c/f5c5a593-319b-4d2b-9ea3-2e5d84e0d782/image)